
Approximately 11 months after the viral sequence was published, two mRNA-based SARS- CoV-2 vaccines received emergency approval, demonstrating the potential of the technology. Vaccines for cancer and infectious diseases have proven effective thanks to low dosages, local delivery, and controlled protein expression. Recent advances have been made in cancer, Epstein-Barr virus, HIV, influenza, and Omicron-specific boosters as part of ongoing studies on mRNA-encoded proteins and cellular immunotherapies. In the clinical setting, the emerging landscape holds great promise for revolutionary mRNA-based treatments.
mRNA Cancer Vaccines: History and Recent Advances
Recent developments in medical research have highlighted the potential of mRNA as a viable approach for addressing challenges in healthcare, particularly in combating infectious diseases and cancer (1, 2). This resurgence of interest in mRNA-based therapeutics, which was once deemed impractical, has been fueled by deeper insights into human genetics. With nearly 21,306 genes in the human genome encoding proteins, mRNA serves as an intermediary in protein synthesis. This characteristic makes mRNA an attractive candidate for producing various proteins, including those necessary for immune responses (3). The developmental history of mRNA vaccine is summarized in Fig. 1.
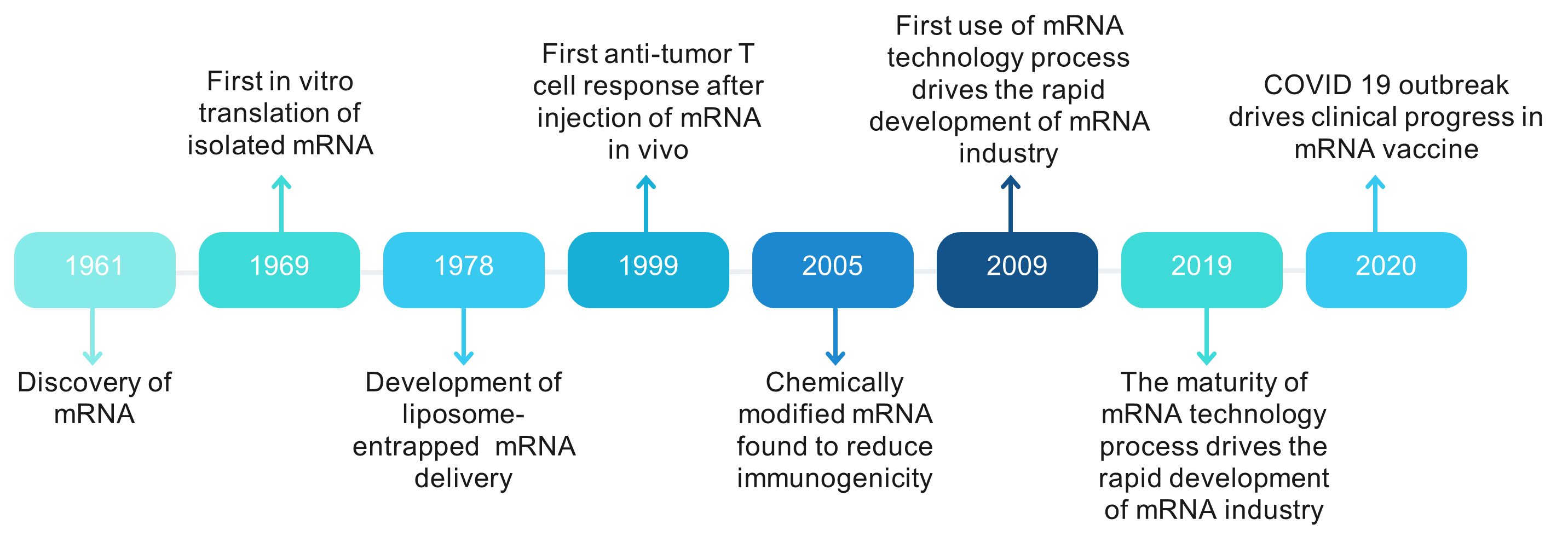
Fig 1. The history of the development of mRNA vaccine.
Unlike traditional genomic engineering methods that involve genome manipulation, mRNA vaccines offer a simpler, more controllable approach. They are easy to produce, manipulate, and deliver into cells, minimizing the risk of unintended genetic alterations. In the context of cancer treatment, mRNA vaccines have shown promise in stimulating the immune system to target tumor-associated antigens and growth factors (4).
The urgency brought about by the COVID-19 pandemic has accelerated research in mRNA therapeutics, leading to significant progress in vaccine development. While traditional vaccine approaches have made significant strides in disease prevention, challenges persist, particularly in developing effective vaccines against cancer. However, previous obstacles in mRNA vaccine research, such as instability and immunogenicity issues, have been overcome with technological advancements and increased research investment. This has positioned mRNA as a versatile tool for innovative therapeutic development, particularly in the field of vaccinology (5).
Advantages of mRNA cancer vaccines
Even though the application of mRNA vaccines in the treatment of cancer is still in its infancy, there are a number of properties of in vitro transcribed mRNA that suggest its potential as a vaccine. Compared to peptide or DNA vaccines, mRNA vaccines provide many advantages that could boost their effectiveness against a larger variety of cancers (6).
Firstly, because RNA-based vaccines can be developed more quickly and cheaply than traditional vaccinations thanks to sophisticated industrial settings and high yields of in vitro transcription reactions. For instance, the first volunteer in a phase 1 clinical study in 2020 received the COVID-19 mRNA vaccination within ten weeks following the discovery of the viral genome sequence (7). Two innovative mRNA vaccines were awaiting authorization as potential COVID-19 vaccines by November 2020. These were mRNA-1273 from Moderna and BNT162b2 from a cooperation between BioNTech and Pfizer (6).
Secondly, the absence of pathogen particles or inactivated pathogens in mRNA vaccines lowers the possibility of unwanted immune reactions. In contrast to DNA cancer vaccines, mRNA vaccines are delivered through a non-integrating platform that is only necessary for it to exist in the cytoplasm. By doing this, the chance of infection, insertional mutagenesis, and the integration of foreign genes into the host genome are all eliminated (6, 7).
Thirdly, early clinical trials have shown that mRNA vaccines are well tolerated by healthy persons and very effective at inducing a consistent immune response. In vitro transcription has made it feasible to synthesise mRNA efficiently in a cell-free environment (8). Additionally, advancements in in vivo delivery have enabled the formulation of mRNA into carrier molecules such as nanoparticles, which accelerate uptake and expression in the cytoplasm. Furthermore, self-amplifying RNA or replicon RNA derived from the genetic backbone of an alphavirus can create a robust immune response by replacing viral structural proteins with a transgene encoding the vaccination antigen (9).
Moreover, personalized medicine, based on an individual's unique genetic makeup, has opened new possibilities for disease prevention and treatment. mRNA-based personalized cancer vaccines, utilizing tumor-specific antigens (TSAs) or tumor-associated antigens (TAAs), can potentially tailor therapy for better patient care based on the individual susceptibility to each disease (10).
Recent innovations in mRNA vaccine technologies
A poly (A) tail, coding region, 3′UTR, 5′ cap, and 5′ UTR make up the basic structure of mRNA. The 5′UTR or 5′ caps control cap-dependent translation initiation, which is essential for efficient protein synthesis. For improving protein translation and maintaining mRNA stability, the 3′UTR—which has an ideal poly (A) signal—is essential. The creation of proteins, the stability and abundance of mRNA, and codon optimisation are all necessary for the successful production of RNA vaccines (11). Traditional technologies, like modified nucleoside incorporation and coding sequence optimization, have been breakthroughs in mRNA vaccine production but can be complex, expensive, or challenging to apply in humans. Hence, there's a need for innovative technologies to efficiently produce mRNA vaccines. Three significant recent innovations in mRNA vaccine technology include modifying mRNA structural elements, optimizing manufacturing platforms, and developing delivery systems (12).
Modification of mRNA structural elements
Optimising the design of mRNA vaccines requires structural improvements because the fundamental structure of mRNA affects translation rate and transcript half-life. The attachment of the 5′ cap to eukaryotic initiation factor 4E (eIF4E), which acts as a bottleneck in mRNA translation, represents a crucial stage in translation (13). While recombinant vaccinia virus can cap mRNA through enzymatic means, synthetic cap analogs exist as alternatives, although they are not as effective. A method to improve translational efficacy and half-life has been investigated that is reverse capping (14).
Endogenous untranslated sections (UTRs) and de novo design have been combined in recent works to engineer mRNA UTRs for enhanced protein synthesis. NASAR UTRs, or the ideal combinations of 5′ and 3′ UTRs, have been found through bioinformatics research and design. mRNA encoding the receptor-binding domain (RBD) prepared with NASAR UTRs demonstrated efficacious immunisation in animal models; In terms of eliciting antigen-specific antibodies, intramuscular injection demonstrated a five-fold greater effectiveness compared to subcutaneous injection (15).
A distinct RNA vaccine approach has shown robust antigen expression and immune response by utilizing a trans-amplifying RNA split-vector system derived from alphaviral self-amplifying RNA. By eliminating the replicase of alphaviral self-amplifying RNA, a transreplicon is created, possessing dose-independent properties. This transreplicon can effectively induce protective immune responses even at minimal concentrations (16).
The poly (A) tail's ideal length is necessary for both effective translation and the longevity of mRNA molecules. It is best to incorporate poly (A) tails of about 100 nucleotides when producing mRNA therapies. Polyadenylation techniques that are both reliable and practical are essential for mRNA therapeutic applications. In one study, a straightforward method for creating and maintaining poly (A)-encoding DNA sequences for in vitro transcription (IVT) of mRNA was described. This method used type IIS restriction enzymes. This method extends the homopolymeric sequence of circular plasmids, which act as templates for mRNA transcription, by repeatedly undergoing asymmetric cleavage, ligation, and propagation. Furthermore, in vitro transcribed transcripts' poly (A) tail performs well in vivo (17).
Optimization of mRNA manufacturing platform
A well-established manufacturing platform is essential for producing therapeutic-grade mRNA, although specific quality attributes are not currently challenging to achieve. Upstream processing, which includes the enzymatic synthesis of mRNA, and downstream processing, which concentrates on mRNA purification, are both components of the mRNA production process (18).
In upstream processing, enzymatic reactions for mRNA generation are less time-consuming than conventional methods. However, the use of cap analogues during the enzymatic reaction increases production costs, hindering large-scale manufacturing. An alternative strategy, CleanCap®, simplifies and reduces production costs by co-transcriptionally adding a natural 5′cap 1 construct during mRNA generation without competing with guanosine triphosphate (19).
Downstream processing involves removing impurities to enhance mRNA performance. For the purpose of eliminating aberrant mRNA species such double-stranded RNA (dsRNA) and truncated RNA fragments, standard purification techniques utilised in lab settings, such as DNase digestion and lithium chloride (LiCl) precipitation, are inadequate (20).
Development of mRNA delivery systems
Several challenges hinder the delivery of mRNA into the cytosol for antigen expression, including the negative charge shared by mRNA molecules and cell membranes, the relatively large size of mRNA molecules, and their susceptibility to degradation by ribonucleases present in the skin and blood. To address these obstacles, various techniques and carriers have been developed for mRNA delivery, such as conjugation with lipid-based materials, polymers, or peptides as delivery vehicles, as well as utilizing naked mRNA transport systems (21).
The Future of mRNA Cancer Vaccine
mRNA-based cancer vaccines represent a promising approach with several advantages, including versatility, potency, scalability, precision, cost-effectiveness, and the ability to be stored without a cold chain. These vaccines may be produced in large quantities for clinical application and address problems related to DNA vaccines (22). However, issues such as instability, intrinsic immunogenicity, and inefficient in vivo administration need to be solved for sustainable and cost-effective manufacture. Modification of mRNA structure, purification methods, and innovative formulation approaches can enhance mRNA internalization by antigen-presenting cells (APCs) and improve delivery efficacy (23). The immune response elicited by mRNA-based cancer vaccines involves antibody production, B cell-mediated humoral reaction, and CD4+/CD8+ T cell response.
Three forms of RNAs are now employed as cancer vaccines: non-replicating unmodified self-amplifying mRNAs (SAMs), modified SAMs, and virus-derived SAMs. With reduced vaccination doses, self-amplifying mRNA (SAM) vaccines, derived from positive single-stranded mRNA viruses, have the capability to self-amplify and induce a robust and long-lasting immune response. However, their clinical application in cancer treatment is currently limited to the early detection of viral replication particles. To target tumor-specific mutations, personalized neoantigen-based cancer vaccines hold promise in enhancing the host's anti-tumor immune response while minimizing adverse effects (24).
The instability of vaccine formulations poses challenges for distribution, especially in remote areas. Cold chain systems are commonly used for vaccine storage and distribution, but alternative methods like lyophilization and spray drying are being explored for long-term preservation. These methods have limitations, such as the need for reconstitution and potential reduction in potency.
Overall, while mRNA cancer vaccines hold significant promise for anti-tumor efficacy, addressing issues related to immunogenic platforms, antigens, adjuvants, delivery materials, administration strategies, and direct comparative studies are critical for future research and development (25).
Conclusion and Future Perspective
In conclusion, the COVID-19 pandemic has made great progress towards creating mRNA vaccines against infectious diseases and cancer. Most cancer vaccines are therapeutic, whereas most vaccinations against infectious diseases are preventative. The FDA has only approved two prophylactic cancer vaccines to prevent virus-induced hepatocellular carcinoma and cervical cancer, respectively. These vaccines target HBV and HPV.
Therapeutic cancer vaccines work as systemic immunotherapies, boosting anti-tumor immunity through the expansion and activation of CD4+ and CD8+ T cells that are specific to antigens. Because mRNA-based cancer vaccines may encode numerous antigens at once and boost both humoral and cellular adaptive immune responses, they are more effective than other forms of cancer vaccines. Furthermore, well-developed mRNA vaccine formulation platforms and manufacturing techniques allow for the quick and widespread manufacture of cancer vaccines.
With more accurate and powerful anti-tumor effects, the use of personalised mRNA-based cancer vaccines has been bolstered by the advent of neoantigens. The ultimate objective of mRNA cancer vaccines is to reduce side effects while increasing efficacy. Despite all of the benefits that mRNA vaccines offer, further advancements in manufacturing techniques and a better comprehension of the various mRNA vaccine types' mechanisms of action are required to reach this objective (26).
References
Disclosures: There is no conflict of interest and disclosures associated with the manuscript.